Bexion Pharmaceuticals, Inc. to Present at the 2021 Association for Cancer Immunotherapy (CIMT) Annual Meeting
FOR IMMEDIATE RELEASE [Covington, KY—April 20, 2021]
Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, announced today that an abstract submitted for an eTalk Session at the CIMT annual meeting to be held virtually May 10-12, 2021 has been chosen for presentation.
Every year, the CIMT Annual Meeting connects the global cancer immunotherapy community in the heart of Europe. As the largest meeting focused on cancer immunotherapy research and development in Europe, CIMT invites international participants for high-level scientific exchange, collaboration, and education focused on cancer immunotherapy.
Title of the Abstract:
“BXQ-350, a first-in-human nanovesicle formulation of the lysosomal protein Saposin C, reprograms the tumor microenvironment and synergized with Immune Checkpoint Inhibitors.”
ETalk Session:
Monday, May 10, 2021 2:00-3:00 pm Central European Summer Time (CEST), 8:00-9:00 am (EST).
Gilles Tapolsky, Ph.D., M.B.A., Vice President, Pharmacology at Bexion will present preliminary preclinical and clinical results of the Phase 1 study that demonstrate stimulation of the adaptive and innate immune systems and the impact of specific immune cells populations as a new way to revive the immune system.
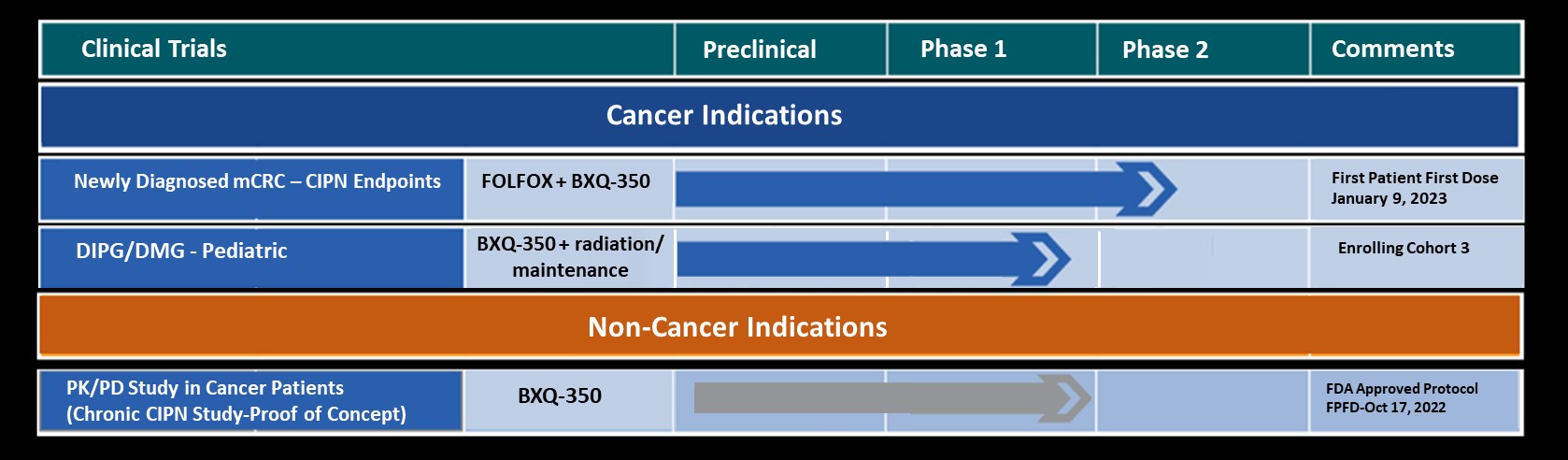
Bexion is developing BXQ-350, a novel biologic, in several cancer indications including Glioblastoma Multiform, Diffuse Intrinsic Pontine Glioma and Colorectal Carcinoma. BXQ-350 has a unique and innovative mechanism of action that inhibits immunosuppressor signaling and stimulates immuno-+effector cells.
About Bexion Pharmaceuticals
Bexion Pharmaceuticals, a clinical-stage biopharmaceutical company, is pioneering the development of life-changing treatments by leveraging the untapped mechanisms of the lysosome. Bexion believes the lysosome is an underexploited cellular orchestrator involved in multiple diseases. Bexion’s lead drug candidate is BXQ-350, a first-in-class biologic containing the multifunctional, lysosomal activator protein, Saposin C and a phosphatidylserine.
BXQ-350 has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in brain and other solid tumors, including those that may lead to brain metastases. Bexion has completed a multi-site first-in-human Phase 1 clinical trial of BXQ-350 for solid tumors and gliomas. Bexion is in Phase 2 for a rare pediatric brain tumor and plans to initiate two adult Phase 2 programs in 2021. Additionally, other clinical and non-clinical data suggest BXQ-350 has activity in CNS diseases, including peripheral neuropathy.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
About the Association of Cancer Immunotherapy
The Association for Cancer Immunotherapy (CIMT) is a member-based information and education platform that facilitates the knowledge exchange between academic and industry scientists, physicians and regulatory authorities who research and develop cancer immunotherapies. CIMT was founded in 2002 by physicians and researchers from different fields of clinical and theoretical medicine as an independent non-profit organization. Every year, CIMT organizes the largest and most influential international cancer immunotherapy meeting in Europe.
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward-looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.