Bexion Pharmaceuticals, Inc. to Present at the 2022 International Symposium on Pediatric Neuro-Oncology (IPSNO) Annual Conference
FOR IMMEDIATE RELEASE [Covington, KY—June 13, 2022]
Bexion Pharmaceuticals, Inc., a mid-stage clinical biopharmaceutical company, announced today that two abstracts submitted for an in-person session at the IPSNO annual meeting to be held in Hamburg, Germany, June 12-15, 2022 has been chosen for poster presentation.
ISPNO is the largest international scientific meeting of multidisciplinary professionals in research, diagnosis, treatment, and rehabilitation of children and adolescents with tumors of the Central Nervous System. The program will include the most recent cutting-edge preclinical and clinical research from the fields of neurosurgery, neuroradiology, neuropathology, biology, radiotherapy, pediatric neurooncology,
immunotherapy, late effects, rehabilitation, and nursing. ISPNO will also have state-of-the art symposia, interdisciplinary sessions and round tables on the most challenging and timely topics in the field of pediatric neuro-oncology.
Title of the Abstract-Poster Presentations:
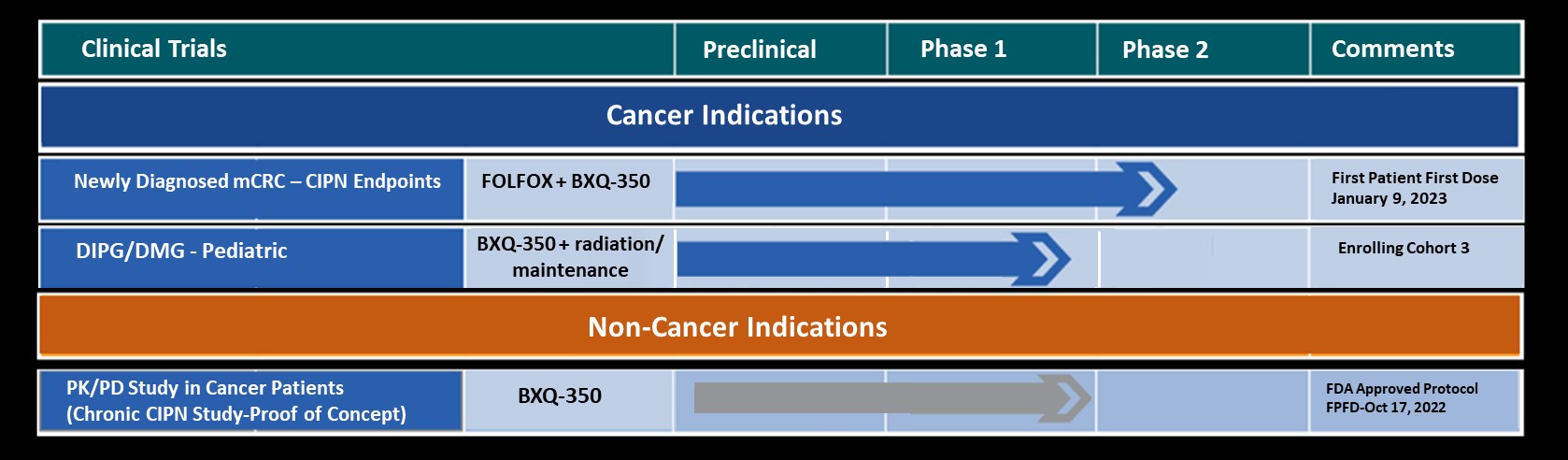
A Phase 1, Safety and Dose Escalation Study of BXQ-350 in Combination with Radiation in Pediatric Patients with Diffuse Midline Glioma or Diffuse Intrinsic Pontine Glioma
A Phase 1, Safety and Dose Escalation Study of BXQ-350 in Patients with Advanced Solid Malignancies Demonstrates that BXQ-350 is Well Tolerated and Shows Signs of Potential Clinical Activity in Ependymoma Patients
Poster Presentation Session:
Monday, June 13th, 2022 from 6:00 – 7:30 pm Central European Summer Time (CEST)
Tuesday, June 14th, 2022 from 5:30 – 7:00 pm Central European Summer Time (CEST)
Gilles Tapolsky, Ph.D., M.B.A., Vice President, Pharmacology at Bexion will be discussing the key findings from these poster presentations.
About Bexion Pharmaceuticals
Bexion Pharmaceuticals, a mid-stage clinical stage biopharmaceutical company, is developing a new generation of biologic immunotherapy to treat solid tumor cancers and Chemotherapy Induced Peripheral Neuropathy (CIPN) with potential portfolio expansion opportunities in other cancers and broader neuropathic pain indications. Bexion’s lead drug candidate is BXQ-350, a first-in-class biologic containing the multifunctional, lysosomal activator protein, Saposin C and a-phosphatidylserine. BXQ-350, an “S1P Activator”, has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in colorectal, brain and other solid tumors. Bexion has completed two single agent Phase 1 programs in adults and in a pediatric population. The Phase 1 programs demonstrated a strong safety profile with evidence of single agent activity across a range of solid tumors including Glioblastoma Multiforme (GBM), colorectal cancer and appendiceal cancer. Additionally, other clinical and non-clinical data suggest BXQ-350 improves symptoms associated with CIPN.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements, including, without limitation, statements related to Bexion Pharmaceuticals Inc.’s (the “Company”) goals, priorities, growth opportunities, new products and solutions, milestones and current and pending clinical trials. All statements contained in this presentation, other than statements of historical fact, are forward-looking statements. The words “anticipate,” “plan,” “estimate,” “expect,” “intend,” “will,” “should,” “forecast,” “project” and other similar expressions are intended to identify forward-looking statements.
These statements are based on management’s current expectations and beliefs. These expectations and beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. By their nature, forward-looking statements involve known and unknown risks, delays, uncertainties, assumptions and other factors because they relate to events and depend on circumstances that will occur in the future, whether or not outside the control of the Company. These factors include results of current or pending clinical trials, risks associated with intellectual property protection, actions by the FDA/HPB/MHRA and other factors. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated, expressed or implied in the forward-looking statements. You should not place undue reliance on forward-looking statements. The Company does not undertake an obligation to update the forward-looking statements, except as required by applicable law.