Bexion Pharmaceuticals Receives Study May Proceed Letter from FDA for a Phase 1b/2 Clinical Trial of BXQ-350 in Newly Diagnosed Metastatic Colorectal Carcinoma
FOR IMMEDIATE RELEASE Covington, KY, 29 September 2021
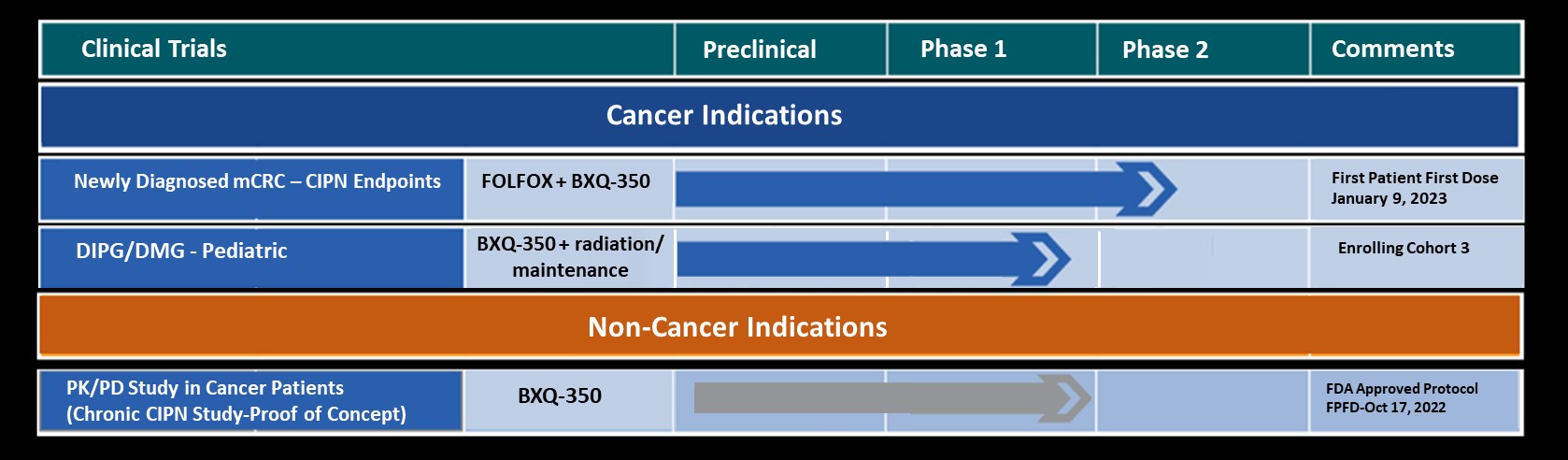
Bexion Pharmaceuticals, Inc. announced today that a Study May Proceed letter has been received from the US Food and Drug Administration (FDA) for the Investigational New Drug Application (IND) for BXQ-350 to initiate a Phase 1b/2 clinical trial in newly diagnosed stage 4 metastatic colorectal cancer patients.
Bexion recently completed Phase 1 studies in both adults and children with advanced solid tumors utilizing BXQ-350 as monotherapy. These studies demonstrated that BXQ-350 has a tolerable safety profile with no dose limiting toxicity (DLT) at the highest administered dose and showed in some patients preliminary evidence of anti-tumor activity (including in advanced colorectal cancers).
Additional pre-clinical and anecdotal data suggest that BXQ-350 may play a role in decreasing oxaliplatin-induced sensory neurotoxicity, a common result of colorectal cancer standard of care.
“BXQ-350 with its proven safety profile, potential efficacy and possible neuropathy benefit makes it a worthwhile candidate to use in combination with standard of care treatment for metastatic colorectal cancer (mCRC), stated Dr. Ray Takigiku, CEO and President of Bexion. “We are hoping to not only enhance the standard treatment of mCRC, but to potentially alleviate the side effects related to oxaliplatin-induced sensory neurotoxicity.”
About Bexion Pharmaceuticals
Bexion Pharmaceuticals, a clinical-stage biopharmaceutical company, is pioneering the development of life-changing treatments by leveraging the untapped mechanisms of the lysosome. Bexion believes the lysosome is an underexploited cellular orchestrator involved in multiple diseases. Bexion’s lead drug candidate is BXQ-350, a first-in-class biologic containing the multifunctional, lysosomal activator protein, Saposin C and a phosphatidylserine.
BXQ-350 has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in brain and other solid tumors, including those that may lead to brain metastases. Bexion has completed a multi-site first-in-human Phase 1 clinical trial of BXQ-350 for solid tumors and gliomas. Bexion is in Phase 1 for a rare pediatric brain tumor and plans to initiate two adult Phase 2 programs in 2021-2022.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward-looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.