Investors
BXQ-350 provides a value proposition that is possibly unprecedented
in how it may address significant unmet medical need across these patient populations suffering from devastating malignant disease.
BXQ-350 is a Phase 2 clinical-stage, novel, first-in-class biologic
that has a unique mechanism of action, sphingolipid metabolism modulation, thereby providing the potential for anti-tumor efficacy across a variety of advanced solid tumors paired with anti-neuropathy effect in patients suffering from CIPN (chemotherapy induced peripheral neuropathy).
Outcomes in mCRC remain dismal despite the use of Standard of Care (SOC) (FOLFOX + bevacizumab). Very few therapies have been approved in first line mCRC in the past decade
In mCRC, mPFS (median Progression Free Survival) is 6-12 months and mOS (median Overall Survival) is 24-32 months with SOC.
FOLFOX is a highly toxic chemotherapeutic regimen based on an Oxaliplatin backbone and forms part of SOC in mCRC. Oxaliplatin is commonly associated with Chemotherapy Induced Peripheral Neuropathy (CIPN), a major dose-limiting toxicity (DLT) which interrupts treatment via reduction in dose, skipping a dose or removal of Oxaliplatin in these patients.
Pipeline in a product
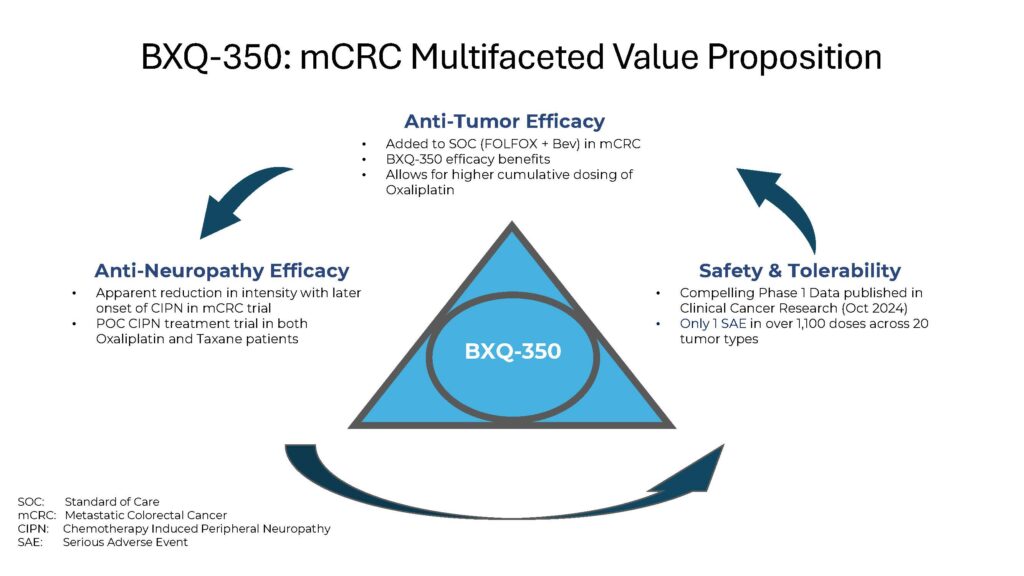
BXQ-350 is the first in a new class of therapies
that has the potential to address unmet medical needs in terms of anti-tumor efficacy but also in terms of anti-neuropathy effect
Non-cancer indications
While BXQ-350 was developed to target solid tumors specifically, early efficacy in neuropathy has emerged through research, primarily for Chemotherapy-induced Peripheral Neuropathy (CIPN)

CIPN is a serious clinical challenge caused by a substantial number of cytotoxic drugs, including taxanes, platinums, vinca alkaloids, bortezomib and some ADCs (Antibody Drug Conjugates.)
CIPN afflicts between 30% and 40% of patients undergoing chemotherapy. Recent studies put the prevalence of CIPN at approximately 68% when measured in the first month after chemotherapy, and 30% at and after 6 months. Debilitating symptoms persist for months to several years after treatment for many of those patients.
BXQ-350 has shown the potential for meaningful reduction of chronic CIPN in a POC (Proof of Concept) study involving cancer patients who are post-chemotherapy Oxaliplatin (n=10) and Taxanes (n=10) in two separate arms.
BXQ-350 has also shown the potential for reduction in CIPN while co-administered with FOLFOX + Bevacizumab SOC in mCRC patients.
This may result in improved QoL (Quality of Life) and improved ability to stay on and sustain chemotoxic therapy in these patients.
Addressing an unmet medical need
BXQ-350 has potentially unprecedented anti-tumor, anti-neuropathy effects that show promise in treating cancer patients whose options for new therapies have historically been limited.
Resources
Find case studies, abstracts, and more about the potential breakthroughs we’re pursuing