Science + Pipeline
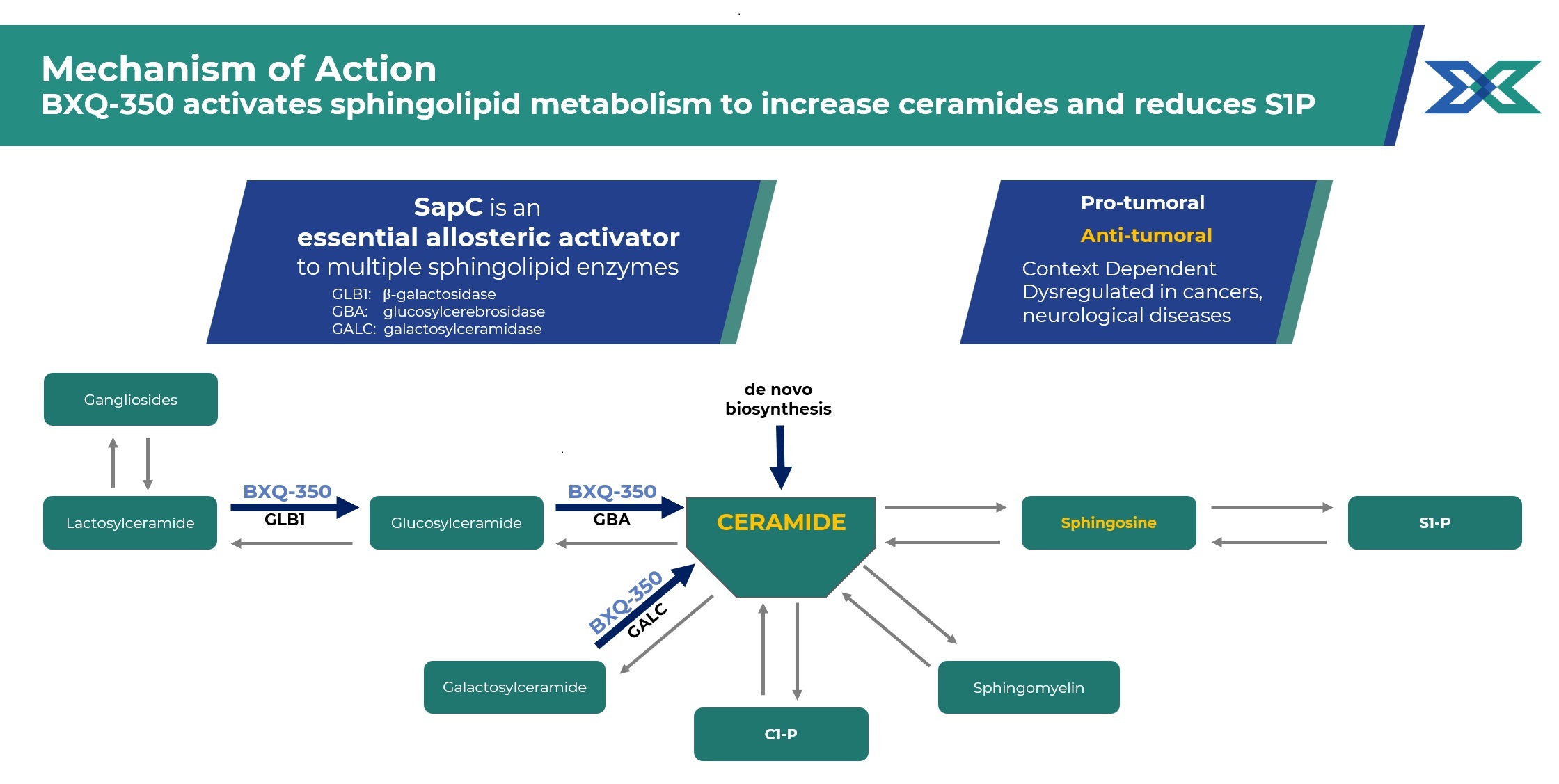
BXQ-350 is a novel sphingolipid metabolism modulator
being developed as a unique biologic for the treatment of solid tumors and chemotherapy-induced peripheral neuropathy (CIPN)
A pipeline in a product
As a modulator of sphingolipid metabolism, BXQ-350 has the potential to address unmet medical needs across a variety of tumor types and CIPN associated with Standard of Care (SOC) chemotherapy. Emerging evidence supports the opportunity for sphingolipid modulation to impact a broad range of indications.
| Clinical Trials | Pre–clinical | Phase 1 | Phase 2 | |
|---|---|---|---|---|
| Cancer indications | ||||
| Newly Diagnosed mCRC BXQ-350 + FOLFOX + bevacizumab |
| |||
| DIPG/DMG – Pediatric BXQ-350 + Radiation |
| |||
| Neuro Indications | ||||
| PoC CIPN Study BXQ-350 Monotherapy |
| |||
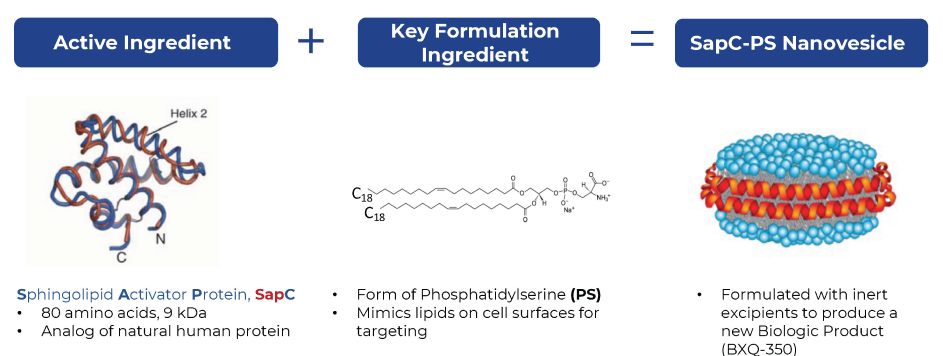
Biologic (SapC)
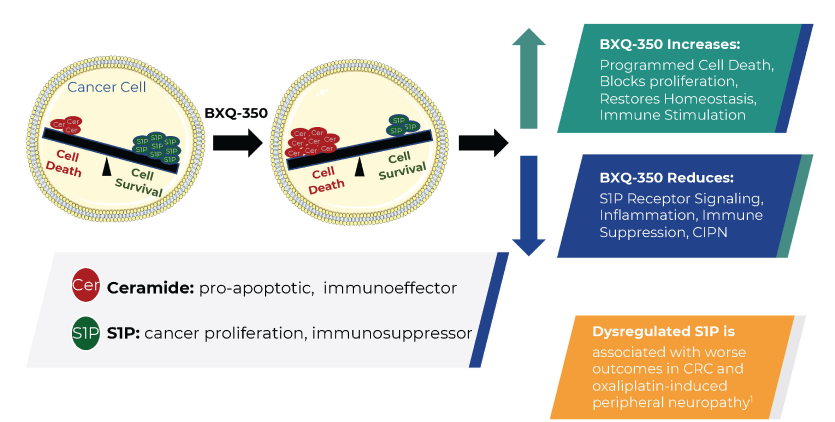
BXQ-350 increases ceramides and decreases Sphingosine 1-phosphate (S1P)
Saposin C
Addressing Significant Unmet Needs in Standard of Care

Sphingolipid metabolism is disrupted by cancers, functioning as a critical survival pathway for tumor cells while also promoting proliferation, migration angiogenesis, and immune evasion.
Scientific and clinical evidence to date suggests that BXQ-350 has the potential to improve cancer treatment for patients through its monotherapy benefits and combination with chemotherapeutics that have neuropathy side-effects by reducing the neuropathy that patients experience, allowing for better quality of life with fewer dose reductions due to neurotoxicity.
Low toxicity in combination treatmentBXQ-350 has the potential to be combined with highly toxic chemotherapeutic agents such as FOLFOX with minimal additional adverse effects.
Neuroprotective propertiesBXQ-350 has the potential to reduce intensity with delayed onset of chronic neuropathy in patients who are either actively receiving chemotherapy or who have previously received chemotherapy.
Chemotherapy-Induced Peripheral Neuropathy
In addition to its potential cancer efficacy, BXQ-350 may address CIPN commonly associated with chemotherapeutics. This creates a unique value proposition of potential safety & tolerability, anti-tumor efficacy and anti-neuropathy effect.
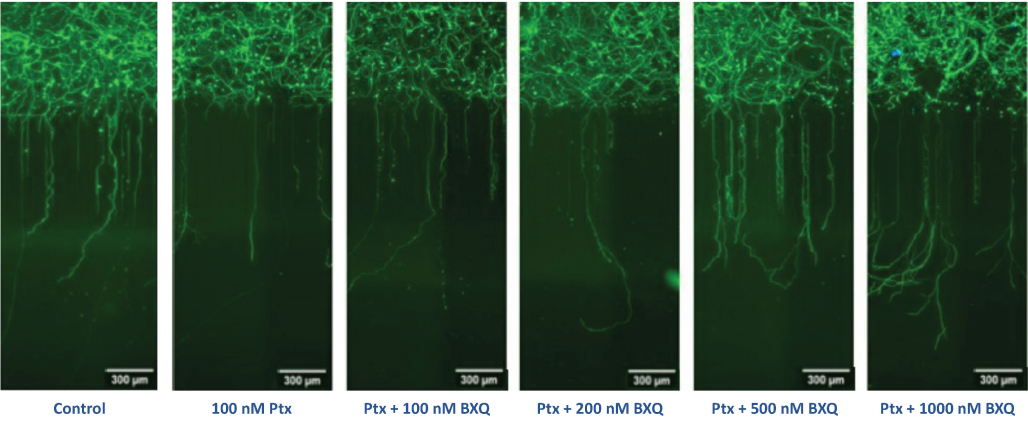
BXQ-350 promotes nerve cell health & growth and reduces damaging effects of chemotherapy.
In Ananda NeuroHTSTM, an imaging assay assessing numerous neuron and axon characteristics, BXQ-350 demonstrates dose-dependent neuron growth and protection against paclitaxel.
Case Studies
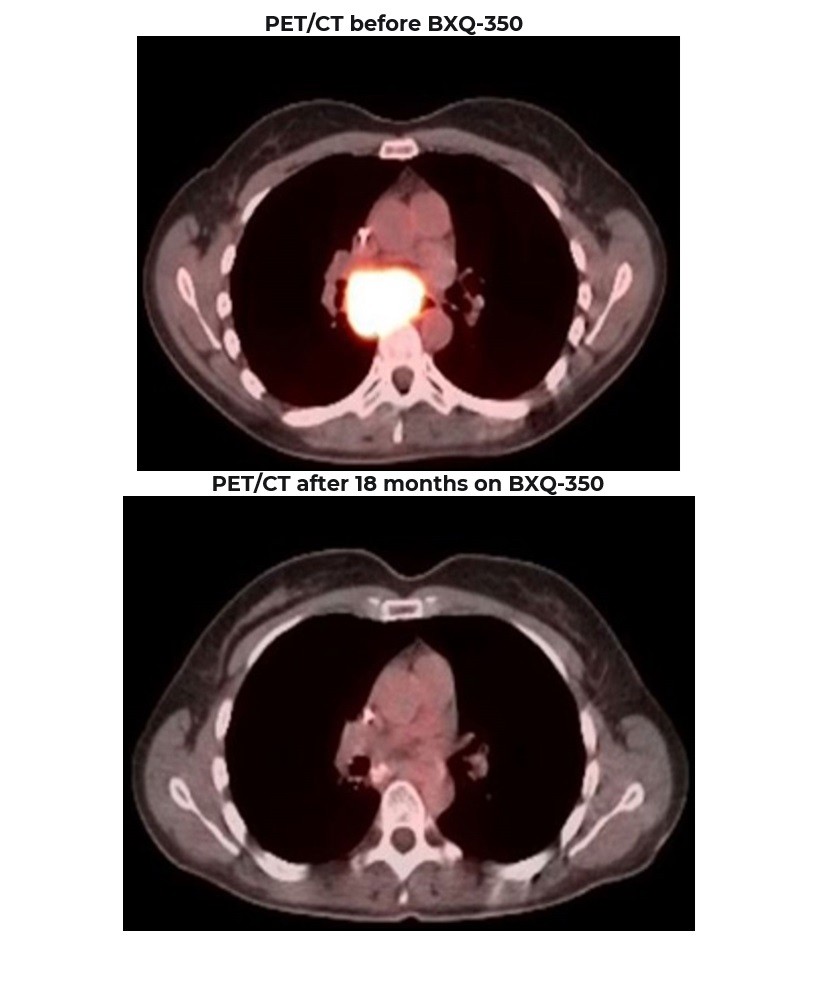
Phase 1 Patient 1080-001: Long Lasting Benefit in mCRC >7 years
Diagnosed in Nov 2015, previously treated with surgery, chemotherapy and radiation (>3 lines)
Rapid progression (5 months) prior to starting BXQ-350
Target lesion (1.5 centimeters) remained unchanged per RECIST criteria
Still Stable Disease over 7 years on study
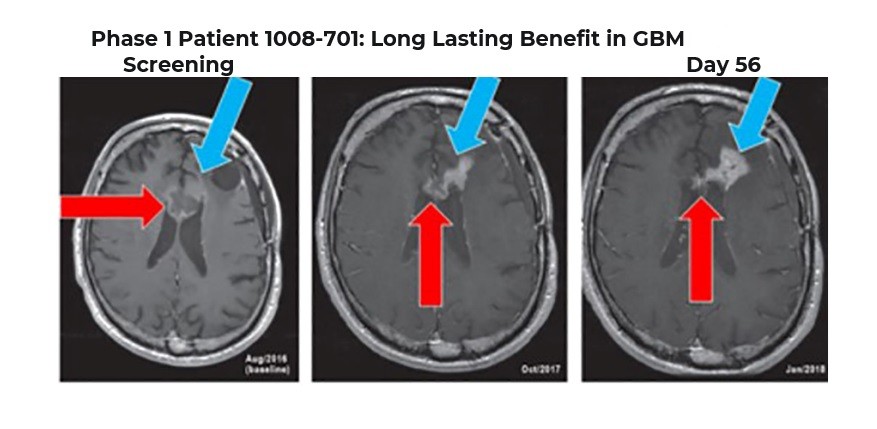
Phase 1 Patient 1008-701: Long Lasting Benefit in GBM > 7 years
Blue Arrows indicate new area of enhancement
Note: Surgery in 2018 revealed significant treatment effect with only trace tumor cells present
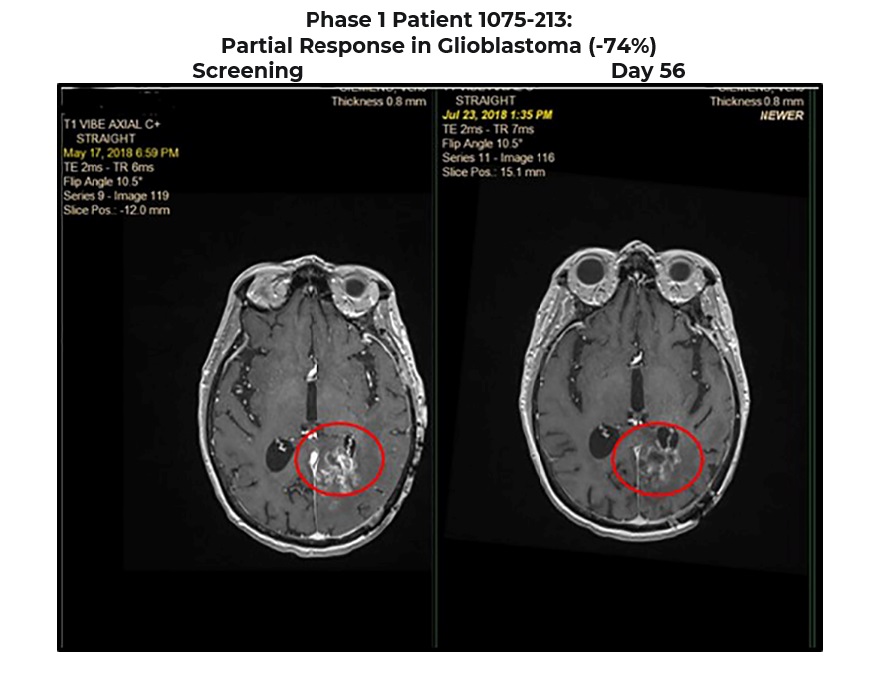
Phase 1 Patient 1075-213:
Partial Response in Glioblastoma (-74%)
GBM diagnosed in 2017 (stage IV) previously treated with surgery, chemotherapy and radiation
Rapid progression (2 months) prior to starting BXQ-350 in 2018
1 target lesion (L parietal) 1.4 centimeters at Screening down to 0.36 centimeter at Day 56 (-74%)
Progressed after 948 days on BXQ-350